This week I started working with anti-body staining. I took two poly di-lysine slides (sample sticks better if coated), segmented tissue, fixed and washed it as I had done with all my previous samples in the weeks earlier. Then I treated it with two 5 minute washes in a 0.1% chemical solution of tween in PBS. This solution is not very harsh and works well at cleaning the sample.
Next, I applied a 0.2% solution of Triton X in PBS to the sample. Triton X permeabilized the membrane, now allowing my “big” antibody proteins into the cell and nucleus. I left the Triton X on the sample for about ten minutes before doing another wash with Tween.
After that, I soaked the tissue in a blocking solution, made up of BSA, for about an hour. This step was to ensure that most protein binding sites were bound to in order to prevent the antibody from targeting anything other than its target site.
The next step was to stain the tissue with the primary antibody, in this case Ki67 Rb (Rb means it was made in a rabbit as a host immune system), and leave it over night. The next day I took the sample out, washed it in Tween again and stained it with the secondary antibody, which had a fluorescent tag attached to it. We used a secondary antibody in this case which targets and binds to the primary antibody. By employing a secondary antibody we are able to bind multiple tags onto the primary antibody and get a stronger signal/visual when imaging the sample under a microscope. Because of the secondary antibody’s photosensitivity, due to the fluorescent tag, I had to carry out this whole part of the experiment keeping the sample covered in tin-foil and away from light.
I also have a picture of what my antibody looked like. It doesn’t look too special compared to the secondary antibody which was a bright neon green color due to the fluorescent tag. Unfortunately I didn’t get a photo of this.
This whole process is the most hands on and “sciencey” thing I felt I have done so far. It was really interesting and took about 3 days until the staining of my sample was complete. On the computational side of things I participated in less coding work this week but I did do a lot of reading and learning about the subject and background information for a coding assignment people in my lab are doing. They are trying to write code that would align an image taken with STORM super resolution, an image only a few microns in size, onto its larger wide field image, thousands of microns in size. This computation would allow scientists to see the general location of their super resolution imaging in a group of cells or what not. They could use coding/imaging techniques such as normalization, blurring, etc., in order to do this.
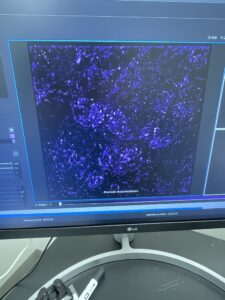
For the first time, I got to image one of my SiR-DNA stained slides on the super resolution microscope. This allowed me to see my sample down at a protein/molecule sized level in a 3D interface. It was extremely fascinating to use a machine and technique that powerful. Here is an image of what my image looked like. The dots on the screen of this image are my SiR-DNA targets, and with further analaysis using this microscope and computer I can see where exactly my target is positioned in a genome and 3D space.
Outside of the lab I had a great week too. On Tuesday I went up to Northern Massachusetts(about an hour and 15 away) with my roommate and his friend to go explore and go on a hike. It was really cool to see the New England countryside. I definitely am missing the woods and mountains a little bit after being in the city for a while.
Yesterday, on Saturday, I also took the train up to Boston and explore that city as well. I went to the aquarium, spent some time exploring the Boston Commons park and Downtown, walked the freedom trail, explored the little Italy neighborhood, and explored the China town neighborhood as well. After a long day of exploring I took the train home back to Providence, which was about an hour and half ride. In this post I included a picture I took from Boston Commons park, the USS Constitutional ship on the Freedom Tour, and the Washington street bridge.








There are no comments published yet.