Hello all! This is the end of my second week working in Professor Baldelli’s lab doing physical chemistry, having roughly three weeks left of this amazing opportunity. During my first week, I was learning the basics of taking contact angles of glass samples coated with Perfluorooctyl vapor (PFOTs). I experimented with two drops, three drops, and four drops of Silane and compared contact angles between those three experiments. I found that four drops of silane computed higher contact angles than three drops or two drops. Building off of that discovery takes place during my second week in research!
After gaining an understanding of contact angles, Professor Baldelli instructed me to conduct two experiments for each drop ratio to ensure consistency in the contact angle measurements. I ended up doing nine experiments total; six without an oven heating the glass before adding the hydrophobic film on it (caused by Silane vapors) and three with the oven just to see if there was a difference. Then I was told to use a program called SciDAVis that lets you create graphs and find experimental errors to help you compare experiment results. The results are as follows:

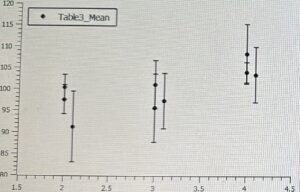
On the x-axis is the number of drops of Silane and on the y-axis is the contact angles measured for each experiment, which addresses the contact angles of both the left and right side of the water droplet on each glass surface. What this graph shows me is a visual difference in contact angles between each experiment, oven or no oven, and the exact pattern of contact angles when increasing the amount of Silane used.
This is an experimental error graph based on the standard deviation and mean of each experiment. To offset oven cleaned glass from no oven cleaned glass I added .1 after the amount of drops used (2.1, 3.1, 4.1). This helped with seeing the differences in contact angles when heating the samples versus not heating the samples before putting them in a vacuum desiccator. Professor Baldelli and I found that by putting the glass substrates in the oven before adding a hydrophobic film on the surface resulted in lower contact angles. Additionally, we observed that with an increase in the amount of Silane used per experiment, the degree of contact angles increased, meaning their relationship is proportional.
Wrapping up this week, my plan for the start of my third internship week is to take contact angles of one drop, five drops, seven drops, and ten drops of Silane to see if the pattern of increasing contact angles repeats as it did with two drops, three drops, and four drops! However, Professor Baldelli told me to clean the glass samples using a mixture of deionized water and soap (what I’ve been doing with all of my glass substrates), then boil them without using the oven afterwards.
The reason why oven cleaned glass produces lower contact angles:
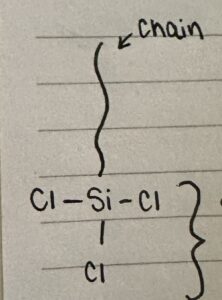
SiCl3 hydrolyzes in the presence of moisture to form silanol groups (Si-OH). The chlorine atoms are replaced by a hydroxyl group (OH), resulting in Si-OH and hydrochloric acid (HCl).
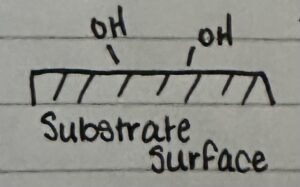
The silanol groups can condense and form strong bonds with the hydroxyl groups already on the glass surface. This is how the hydrophobic film is created.

However, when heating occurs, the OH groups on the glass’ surface breaks down and forms oxygen plus water, which decreases the intensity of hydrophobicity applied to the surface.
Life outside of research this week consisted of celebrating my roommate’s birthday and feeding food begging squirrels!




There are no comments published yet.